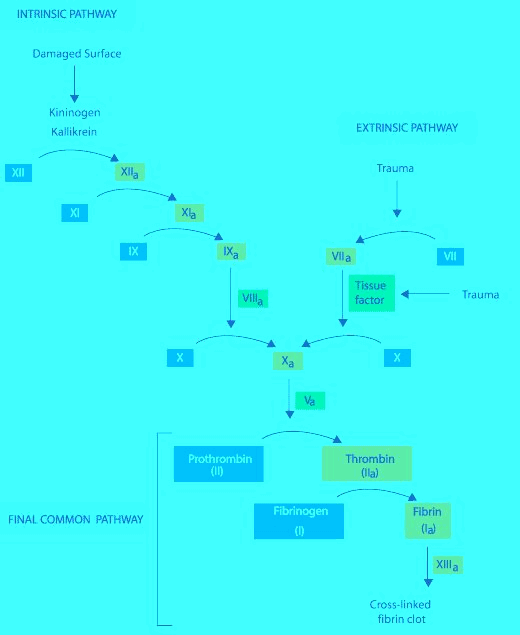
 Diagram of the Classic Clotting Cascade
Vitamin-K Antagonist: Coumarin (Warfarin, Dicumarol and phenprocoumon)- These drugs exhibit their anticoagulant activity by acting as competitve inhibitors of the enzyme (E.C.1.1.4.1) Vitamin-K Epoxide Reductase. Inhibition of the enzyme prevents the reduction of oxdised vitamin-k. Therefore, the Hepatic Gamma-glutaryl-Carboxylase enzyme is starved of an essential co-factor required for its biological activity. HGGC is required to activate Factors 10,9,7,and 2 these factors are essential to the maturation of both the Intrinsic and Extrinsic clotting pathways. Warfarin Therapy, like several other anticoagulant therapy, must be monitored by quantitative measurement methods. Measuring (PT and APTT) followed by (INR) referencing is almost routely done in the clinical setting. Cation Chelators: EDTA, Citrate, Oxalate, Flourine, and Heparin. These organic molecules can serve as Anticoagulation agents in a clinical setting, but the use has been limited. These molecules exhibit their anticoagulant activity by Chelating Calcium and Magnesium ions from the reaction solution. Calcium and Magnesium are essential metal ion co-factors for several enzymatic reactions. These molecules all share a predominantly negatively charged chemical state. These Chelators can also be used to treat Heavy metal ion poisoning. PLASMIN: A Serine Protease involved in the process of fibrinolysis. Fibrin, when activated by Factor-13 forms strong covalent crosslinkages between adjacent firbrin subunits by facilitating the binding of specific residues on the fibrin sub-unit. The crosslinkage can be lysed by the activity of a serine protease called Plasmin. Plasmin is produced by the activity of (Tissue Plasmingen Activator) and (EC.3.4.21.73) Urokinase on inactive Plasminogen. When converted from plasminogen into plasmin, it functions as a serine protease, cutting C-terminal to these lysine and arginine residues. Fibrin monomers, when polymerized, form protofibrils. These protofibrils contain two strands, anti-parallel, associated non-covalently. Within a single strand, the fibrin monomers are covalently linked through the actions of coagulation factor XIII. Thus, plasmin action on a clot initially creates nicks in the fibrin; further digestion leads to solubilization Lysis of Fibrin produces Fibrin Degradative Products or (FDP's). A clinically significant FDP is the D-Dimer protein whose concentration is routely assayed in cases of Deep Vein Thrombosis, Acute Myocardial Infractions, Disseminated intravascular coagulation (DIC), Pulmonary Embolism, and as a reference point when managing cases using Anticoagulants or Fibrinolytic therapy. Auto-regulation of the Fibrinolysis is achieved by the activity of alpha-2 Antiplasmin and Alpha-2 Macroglobulin on Plasmin, and also by the activity of Plasminogen activator 1 & 2 on (EC.3.4.27.73) Urokinase and Tissue Plasminogen Activator. Plasmin activity is also reduced by the activity of Thrombin-Activatable-Fibrinolysis-Inhibitor. Plasmin is also indicated in the lysis of Complement Convertase 3. Direct Thrombin Inhibitors: Members of this drug class include: Hirudin, Bivalirudin, and Argatroban. These drugs work by acting as competitive inhibitors of Factor-2(Thrombin); this is possible by the binding of these drugs to the Active site or the exosite-1 of the Thrombin protease enzyme. Divalent Thrombin Inhibitors: Hirudin & Bivalirudin bind to both the Active site and the exosite, Univalent thrombin Inhibitors: Argatroban & Dabigatran bind to the active site only. Hirudin is a naturally occuring Direct Thrombin inhibitor derived from the Medicinal leech (Hirudo Medicinalis). Direct Thrombin Inhibitor activity is monitored by the ECT assay Ecarin Clotting Time. Ecarin is a compound obtained from snake venom. | The clotting Cascade is an Important biochemical pathway which is required as part of the body's defense against trauma and shock associated with tissue injury.
Blood clots are essential in order to prevent significant blood loss when blood vessels are injured. Blood clotting is an extremely ordered systematic event, and it involves several proteins and essential cations. The cascade is relatively dormant until Tissue Factors or Endothelial Factors are activated.
Diseases that directly affect the clotting cascade include:
Von willibrand Disease,
Haemophilla A (Factor-8 Deficiency),
Haemophilla B (Factor-9 Deficiency),and
Haemophilla C (Factor-11 Deficiency)
The cascade is auto-regulated by the activity of :
Antithrombin-(Directly modulates the activity of Factor-10 & Factor-2),
Thrombin (Factor-2)- (Indirectly modulates the activity of Factor-5 & Factor-8, by activating protein C complexed with Thrombomodulin,and
Factor-3 Pathway Inhibitor(Tissue Factor Pathway Inhibitor)-(directly modulates Factor-7 of the Extrinic Pathway)
The Clotting Cascade has been the target of several anticlotting agents; one such agent is
Heparin- A highly Sulphated Glycoaminoglycan)is highly negatively charged biomolecule. Heparin was initially isolated from the Liver of dogs at the John Hopkins University.
The drugs is often administered if Vitamin K-antagonist are ineffective,or if rapid anticoagulation is required.
The drug works by actively binding to AntiThrombin and Thrombin(Factor-2). The drug indirectly inactivates Factor-10 by the activation of (AntiThrombin) in addition to its direct activity on Factor-2.
The capacity of heparin to effectively bind and inactivate Factors of the clotting cascade is made possible by the size of the Heparin molecule.
Anti-Factor-10 activity by Heparin is facilitated by the a pentasaccharide(sugar units long) binding site which facilitates the binding of AntiThrombin.
The anti-Thrombin activity of Heparin, though, is facilitated by the formation of a Ternary Complex between AT(Antithrombin),Heparin and Thrombin. This requires at least 18 saccharide units.
This size difference has led to the development of (LMWH's)Low Molecular weight Heparin's which can significantly target Anti-Factor-10 activity.
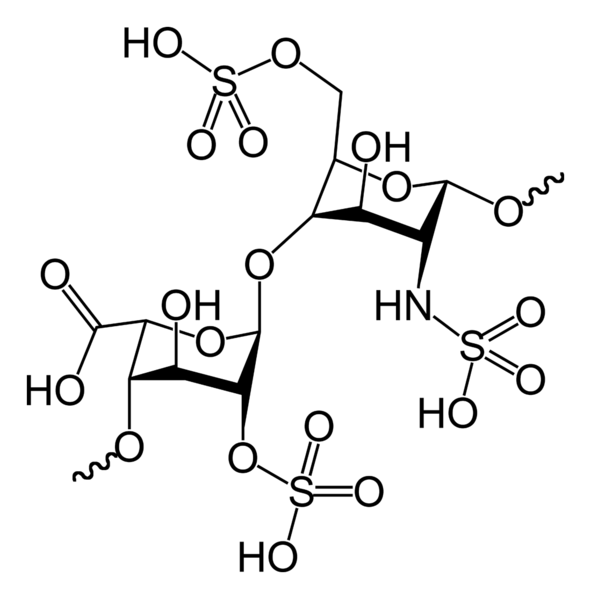 2-D Molecular structure of Heparin
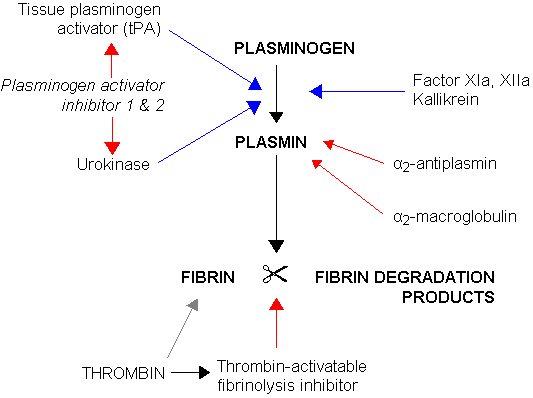 Diagram of Plasmin Actvation & Degradation
|